Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Kumagai, Yuta; Fidalgo, A. B.*; Jonsson, M.*
no journal, ,
Radiation-induced oxidative dissolution of nuclear fuel is anticipated in the geological repository of spent fuel and after severe accidents, where the fuel would be in direct contact with water. Therefore, understanding of the oxidative dissolution of UO is indispensable to estimate radioactive release. This study has addressed effects of hyperstoichiometry of UO
is indispensable to estimate radioactive release. This study has addressed effects of hyperstoichiometry of UO and adsorption of organic compounds on the process. Comparison between hyperstoichiometric UO
and adsorption of organic compounds on the process. Comparison between hyperstoichiometric UO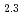 and stoichiometric UO
and stoichiometric UO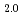 demonstrated that the reactivity of UO
demonstrated that the reactivity of UO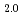 approaches that of UO
approaches that of UO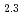 by the H
by the H O
O reaction. The result indicates formation of hyperstoichiometric surface layer during the oxidative dissolution. Phthalic acid, which was used as a model compound, suppressed the U dissolution by
reaction. The result indicates formation of hyperstoichiometric surface layer during the oxidative dissolution. Phthalic acid, which was used as a model compound, suppressed the U dissolution by  -ray irradiation but had little involvement in the H
-ray irradiation but had little involvement in the H O
O reaction, in spite of adsorption exceeding 1 molecule/nm
reaction, in spite of adsorption exceeding 1 molecule/nm . The results suggest an involvement of radical intermediate derived from phthalic acid in the surface reaction.
. The results suggest an involvement of radical intermediate derived from phthalic acid in the surface reaction.